It wasn't until a pig nosed me in the backside, in a friendly way, that I mustered the courage to touch one. I had seen thousands of hogs over the past 18 hours, but I had been nervously keeping my hands to myself. This particular pig seemed to disapprove of my restraint. I scratched him on the crown of his pink, wiry-haired head. He snorted loudly.
I was in a pungent, crowded barn on a farm that raises 30,000 pigs a year in Frankfort, Ind., a sleepy farming town 45 miles northwest of Indianapolis. The farm belonged to Mike Beard, who was standing next to me. The pigs belonged not to Beard but to TDM Farms, a hog production company. Beard has a contract to raise TDM's pigs from when they are 14 days old, just weaned from their mother's milk, until the age of six months, when they are trucked to a processing plant and made into pork chops, sausages and tenderloins. The 40-by-200-foot barn housed 1,100 pigs. Because Beard is paid for the space he provides rather than by the number of pigs, “it's to the company's advantage to keep the buildings as full as they can,” he explained. At 7:30 that evening, a tractor-trailer would deliver 400 more piglets, and as soon as they got settled, Beard planned to give them TDM-approved feed containing antibiotics—a necessity if they were to stay healthy in their crowded, manure-gilded home. Antibiotics also help farm animals grow faster on less food, so their use has long been a staple of industrial farming.
But there is a terrifying downside to this practice, which was one reason I had been hesitant to touch my porcine pal. Antibiotics seem to be transforming innocent farm animals into disease factories. The animals become sources of deadly microorganisms, such as the methicillin-resistant Staphylococcus aureus (MRSA) bacterium, which is resistant to several major classes of antibiotics and has become a real problem in hospitals. The drugs may work on farms at first, but a few microbes with the genes to resist them can survive and pass this ability to fight off the drugs to a larger group. Recent research shows that segments of DNA conferring drug resistance can jump between different species and strains of bacteria with disturbing ease, an alarming discovery. By simply driving behind chicken transport trucks, scientists collected drug-resistant microbes from the air within their cars. Early this year scientists discovered that a gene coding for resistance to a last-resort antibiotic has been circulating in the U.S. and was in bacteria infecting a woman in Pennsylvania.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Many researchers worry—and the new findings add fresh urgency to their concerns—that the abundant use of antibiotics on farms is unraveling our ability to cure bacterial infections. This latest research, scientists now say, shows resistance to drugs can spread more widely than previously thought and firms up links in the resistance chain leading from animal farm to human table. In 2014 pharmaceutical companies sold nearly 21 million pounds of medically important antibiotics for use in food animals, more than three times the amount sold for use in people. Stripped of the power of protective drugs, today's pedestrian health nuisances—ear infections, cuts, bronchitis—will become tomorrow's potential death sentences.
Yet the farm industry argues these worries have been wildly overblown. The idea that antibiotics “in animals directly relates to a risk to human health, we believe, has been greatly exaggerated,” says Richard Carnevale, vice president of regulatory, scientific and international affairs at the Animal Health Institute, a trade group that represents veterinary pharmaceutical companies. Researchers have not directly shown that farm antibiotic use is sparking more resistant infections in people, he and other industry representatives point out. Many of the drug-resistant infections circulating in today's hospitals have never been linked to farms or animal meat.
Scientists now counter that the farm industry is the one exaggerating—even engineering—scientific uncertainty to protect their interests. “Frankly, it reminds me of the tobacco industry, the asbestos industry and the oil industry,” says James Johnson, an infectious disease physician at the University of Minnesota who studies antibiotic-resistant pathogens. “We have a long history of industries subverting public health.” He and other researchers admit that it is difficult to connect all the dots, but the farm industry, they say, deliberately makes it harder. Some big meat companies instruct their farmers to keep researchers away, arguing they need to keep animals free of outsiders and their diseases, which makes it impossible for scientists to solidify the science. As Tara Smith, an epidemiologist who studies emerging infections at Kent State University, tells me, the companies “want us to prove all these steps, but they're really tying our hands.”
I traveled to Beard's farm, as well as two others, in an attempt to find the truth. I decided to follow in the footsteps of scientists who have been trying to trace antibiotic resistance down the long road from farm to food plate to understand whether pigs, cows, chickens or turkeys raised with antibiotics really could bring on the apocalypse—or whether these innocent-looking animals, and the billions of bacteria teeming inside them, are nothing to fear.
Protected Pigs
Eighteen hours earlier I had pulled into the driveway of Schoettmer Prime Pork in Tipton, Ind. The first thing that greeted me was not the sight of pigs or the pungent smell of manure. It was a menacing yellow sign: “WARNING: DISEASE PREVENTION PROGRAM. DO NOT ENTER.”
Because I was there by invitation, I drove in anyway and parked two cars behind a Ford Taurus with the license plate “EATPORK.” Keith Schoettmer, the farm's owner and my tour guide, waved me over from a doorway on my right.
The intimidating sign, Schoettmer explained, was among his careful efforts to prevent pathogens from sickening the 22,000 pigs he raises every year. “The old adage ‘an ounce of prevention is worth a pound of cure’ is never more true than on a pig farm,” said Schoettmer, whose receding white hair and broad smile reminded me of John McCain, although his accent—“manure,” a frequent utterance, was “min-URR”—placed him firmly in the Midwest. Schoettmer asked me to don protective coveralls and plastic shoe covers while we walked around, too, to protect his hogs from any microbes I might be harboring.
Bacteria are everywhere, but they are more everywhere on livestock farms because everybody is literally walking around in poop. (Even though I was covered in plastic the whole time I toured Schoettmer's farm, I reeked when I checked into my hotel room hours later.) And like germs in an elementary school, the bacteria in this excrement get shared widely—they get burrowed under the fingernails of visitors who scratch the animals' heads, and they contaminate the hands of farm employees. (I never saw anyone wearing gloves.)
In 2005 researchers in the Netherlands, which has a large pig industry, determined that livestock-associated strains of MRSA were ailing Dutch pig farmers and their families. MRSA can cause deadly skin, blood and lung infections; it has circulated in hospitals for decades and, more recently, has been affecting people outside of medical settings. By 2007 one fifth of the Netherlands' human MRSA infections were identical to bacteria that had come from Dutch livestock. After this discovery, in 2008, the Dutch government announced strict policies to reduce farm antibiotic use, which then dropped by 59 percent between 2009 and 2011. Denmark, another major pork exporter, had already banned the use of antibiotics in healthy pigs in 1999; in general, Europe has taken a harder line against animal antibiotics than has the U.S.
Now scientists know that this livestock-associated MRSA is spreading throughout the U.S., too. When Tara Smith, then at the University of Iowa, heard what was going on in the Netherlands, she decided to test pigs for MRSA at a few Iowa farms where one of her colleagues, a veterinarian, had connections. “We ended up sampling 270 pigs in the first round—we just went out and swabbed a lot of pig noses and had no idea what we'd find,” Smith recalls. “About 70 percent of them were positive for MRSA.”
Smith and her colleagues have continued to publish a series of disturbing studies showing that MRSA is all over American hog farms. They found MRSA growing in the nostrils of 64 percent of workers at one large farm and found that feed on another farm harbored MRSA even before it got unloaded from the delivery truck. Two hundred thirty-five yards downwind of another farm, Smith found MRSA floating in the air. Other resistant bacteria have been found around poultry farms: after researchers at the Johns Hopkins Bloomberg School of Public Health drove cars, windows down, behind trucks that were transporting chickens in Maryland and Virginia, along the Delmarva Peninsula, they found antibiotic-resistant enterococci—a group of bacteria that causes 20,000 infections in the U.S. every year—in the air inside the cars, as well as resting on the top of soda cans in the cars' cup holder.
Animal poop is used to fertilize crop fields, too, which means that its bacteria are literally spread on the soil used to grow our food. A 2016 study reported that after manure from hog and dairy farms was applied to soil, the relative abundance of antibiotic-resistance genes in the dirt shot up by a factor of four. In a study conducted in Pennsylvania, people who were the most heavily exposed to crop fields treated with pig manure—for instance, because they lived near to them—had more than 30 percent increased odds of developing MRSA infections compared with people who were the least exposed. Beard runs a second business as a manure applicator—he loads 6,500 gallons of his hog manure into a single tanker truck and applies it to nearby fields—and as he noted, the process is tightly regulated. He has to perform soil tests to ensure that fields can absorb the manure nutrients, and he has to apply the manure at a slow enough rate to prevent runoff. But problems can still occur. A 2006 Escherichia coli outbreak in spinach was traced back to crop irrigation water that, investigators believe, had been contaminated by pig and cow manure from a nearby farm. The outbreak killed three people.
Spreading Resistance
Clearly, antibiotic resistance is a problem both for people and for livestock. But how can we be sure that the two are connected and that resistance is exacerbated by on-farm antibiotic use? In 1975 the Animal Health Institute asked this very question and recruited Tufts University biologist Stuart Levy to find out. Levy and his colleagues fed low doses of the antibiotic tetracycline to a group of 150 chickens on a nearby farm that had never gotten antibiotics in their feed and monitored them to see what happened. Within a week, almost all the E. coli bacteria in their intestines were tetracycline-resistant. Three months in, the bacteria growing inside the chickens were also resistant to four other types of antibiotics. After four months, the bacteria growing inside chickens on the farm that had not been fed tetracycline also harbored resistance to the drug. When Levy and his colleagues analyzed the bacteria growing inside the farm owners, they found that 36 percent were tetracycline-resistant, compared with only 6 percent of bacteria from their neighbors. At the time, the findings came as a shock. “The idea that you would be able to give animals antibiotics at low levels and not have them develop resistance was the word of the day, and that made our study that much more interesting and unexpected,” Levy recalls. (The Animal Health Institute has not funded any additional studies to confirm his findings.)
One study reported that more than 90 percent of E. coli in pigs raised on conventional farms are resistant to tetracycline, whereas a whopping 71 percent of E. coli in pigs raised on farms without antibiotics are also resistant. That is because resistance genes spread so well. In a landmark 2012 study, microbiologist Lance Price, now director of the Antibiotic Resistance Action Center at George Washington University's Milken Institute School of Public Health, and his colleagues traced the evolutionary origins of the livestock-associated MRSA that was being shared among pigs and their farmers in Europe and the U.S. by sequencing the whole genomes of 88 diverse MRSA samples. Their findings showed that this MRSA strain started out in people as a methicillin-susceptible form of S. aureus. Then the bacteria jumped into livestock, where they swiftly acquired resistance to methicillin and tetracycline and spread further.
Click or tap to enlarge
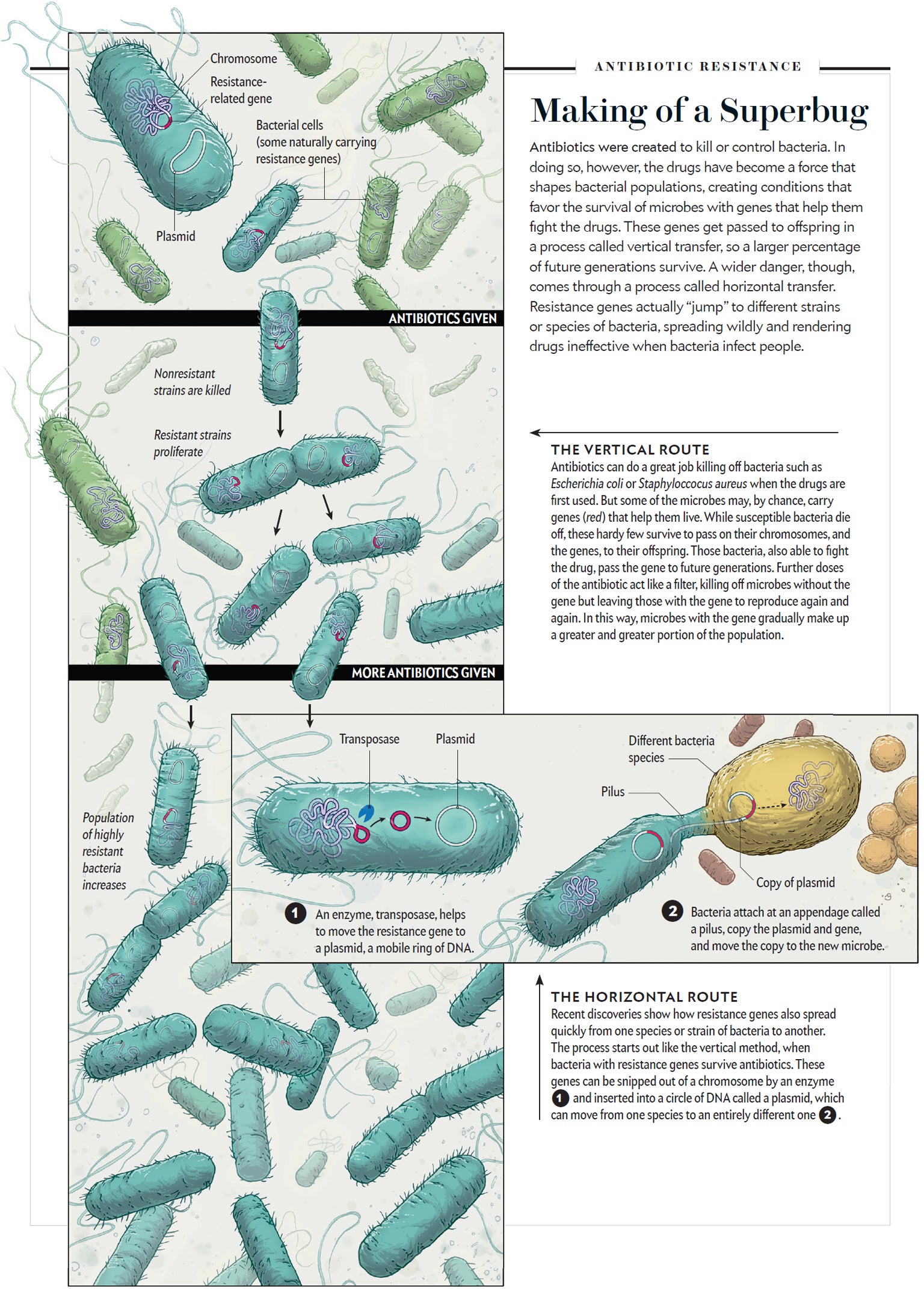
Credit: MATTHEW TWOMBLY
At first, antibiotic resistance spreads slowly and through parent-offspring relationships—the descendants of resistant bacteria are born resistant, too. But emerging research shows that over time, resistance genes find their way onto nimble pieces of DNA that dance around the bacterial genome, and many end up on small circles of DNA called plasmids—copies of which can easily be shared among bacteria of different species. In a 2014 study, a group of international researchers collected samples of antibiotic-resistant E. coli from both people and chickens. Although the bacteria were genetically different, many contained nearly identical plasmids with the same antibiotic-resistance genes. It was the organism-jumping plasmids, rather than the bacteria themselves, that spread resistance.
The fact that resistance can be spread in this way—microbiologists call it “horizontally”—changes everything. It is as if doctors suddenly discovered that Huntington's disease was not just passed down from parent to child but could also infect people who touch one another in passing. It also means that exposing one type of bacteria to one antibiotic in one place has the potential to change how other types of bacteria respond to other antibiotics in other places.
Resistance typically comes at a cost: The mutations draw down the cellular energy a microbe uses to reproduce. Individuals survive, but the whole population grows more slowly. So when bacteria stop being exposed to antibiotics, they ditch their resistance genes over multiple generations. Yet new research suggests that when bacteria are repeatedly exposed to antibiotics, they evolve resistance mutations that let them maintain higher reproductive rates—and then they stay resistant even if antibiotics are taken away. “What's really scary is that we've seen these examples in the gut where sometimes plasmids will transfer from one bacterium to another in a patient, and then they'll rearrange,” says Tim Johnson, a microbiologist at the University of Minnesota College of Veterinary Medicine. “It's like it's evolving in real time in the host to become more efficient.”
Multiple resistance genes also may end up on the same plasmid, so when one gene gives bacteria a survival advantage, other resistance genes come along for the ride. The extent of this co-selection, as it is called, is still a mystery; there is likely to be a lot “that we're not yet even aware of,” Tim Johnson says. Yet figuring it out will be crucial for understanding how resistance spreads and how it could threaten us. Some of the antibiotics used by the farm industry are rarely or never used in humans, and the assumption—often touted by industry—is that resistance that develops to these nonhuman drugs will not pose a risk to people. But co-selection means that the use of one antibiotic could “select for resistance in another,” according to Scott McEwen, an epidemiologist who studies antibiotic resistance at the University of Guelph's Ontario Veterinary College. Growing levels of resistance to a farm antibiotic may also increase levels of resistance to, say, penicillin.
Making matters worse, new research suggests that when bacteria get exposed to antibiotics, they share their resistance plasmids at a faster rate. It is as if the microbes band together in the face of a common enemy, sharing their strongest weapons with their comrades. And once bacteria become resistant, the presence of antibiotics only makes them more successful. One reason that resistant infections are so common in hospitals is because antibiotic use there is so common: the drugs kill off susceptible bacteria yet allow the resistant bugs, suddenly devoid of competition, to thrive—making it easier for them to contaminate medical equipment, staff and other patients.
Government Counterattack
In the face of these terrifying observations, one might think the U.S. government is cracking down on agricultural antibiotic use. It is—kind of. The Food and Drug Administration released two voluntary recommendations in 2012 and 2013—the agency calls them “guidances”—that will be phased in by January 2017. In them, the agency has asked veterinary pharmaceutical companies to change the labels of their medically important antibiotics to say they should no longer be given to animals just to help them grow larger on less feed. The guidances also ask companies to stop selling feed- or water-grade antibiotics over the counter, requiring prescriptions from veterinarians instead.
Most companies have agreed to comply with the suggested rules. The problem is that a lot of livestock farms, including Schoettmer's and Beard's, say they stopped using antibiotics for growth promotion a long time ago. Their main reason for using antibiotics now, they say, is for “disease prevention and control,” a purpose that will not be affected by the new rules. As long as their vets agree, farmers will still be able to mass-treat their animals with antibiotics when they fear that they may be vulnerable to infection. “I think you'll find [this use] relatively normal in the industry,” says Schoettmer, who in 2015 was crowned America's Pig Farmer of the Year by the National Pork Board. (The board was created by Congress to promote the industry and is overseen by the U.S. Department of Agriculture.) He notes that the goal is “to make sure any of these very common pathogens don't get a toehold and start to make these pigs suffer.”
According to 2012 USDA data, almost 70 percent of American hog farms mass-feed antibiotics to their animals to prevent or control the spread of disease; nearly all give their pigs antibiotic-laced feed at some point in their lives. Likewise, more than 70 percent of cattle raised on large U.S. feedlots are fed medically important antibiotics, and between 20 and 52 percent of healthy chickens get antibiotics at some time as well. Yet farmers who contract with big companies may not even know when they are giving antibiotics, because they are provided with pretreated feed. When I asked Beard at what ages his pigs were given antibiotics, he said he had to contact TDM to find out.
It makes sense that animals on crowded industrial farms need antibiotics; the conditions of their lives leave them vulnerable to disease. “Density makes it more difficult to eliminate pathogens, and the risk of infection is greater,” says Steve Dritz, a veterinarian at Kansas State University. The pigs I saw were crawling and lying on one another; some were snoozing in or nosing around in feces. U.S. livestock farms have been exploding in size in recent decades: in 1992 only 30 percent of farms raised more than 2,000 hogs at a time, but by 2009 farms this large accounted for 86 percent of the country's hog industry—in large part because so many small farms went out of business. There is a lot of economic pressure on these farmers. Hog prices have dropped. Companies that contract with poultry farmers insist the farms regularly upgrade their already expensive equipment and bear the cost. In 2014 only 56 percent of intermediate-sized farms reported any actual income from their farming work.
With this setup, “farmers basically have to have perfect management and perfect environments—perfect everything to keep disease out. Otherwise, they lose their flocks,” Tim Johnson says. “It's not the farmer's fault, because the industry has pushed them toward this.”
Links in the Sausage Chain
The morning after I toured Schoettmer's farm, before leaving for Beard's, I went down to the buffet breakfast at my hotel. I paused in front of the sausage: Had any of it come from Schoettmer's pigs? He sells most of his hogs to Indiana Packers Corporation, which processes and sells the pork to local retailers. It was possible that the patties in front of me were made from some of his animals.
I took one, albeit reluctantly. What were the chances, I wondered, that this meat would give me a resistant infection? When livestock are slaughtered, their meat can get splashed with bacteria from their intestines. In a 2012 study, FDA scientists analyzed raw retail meats sold around the country and found that 84 percent of chicken breasts, 82 percent of ground turkey, 69 percent of ground beef and 44 percent of pork chops were contaminated with intestinal E. coli. More than half of the bacteria in the ground turkey were resistant to at least three classes of antibiotics. These microbes can cause food poisoning if meat is not cooked properly before it is eaten or if a person handling the raw meat does not wash his or her hands properly afterward.
But new research suggests that foodborne pathogens can make us sick in other ways, too. Price and his colleagues study strains of E. coli that he calls COPs—colonizing opportunistic pathogens. As he outlined in a 2013 paper, these bacteria most likely get inside people via food but do not, at first, cause illness; they simply colonize the gut, joining the billions of other “good” bacteria there. Later, they can infect other parts of the body, such as the urinary tract, and cause serious illness. Urinary tract infections among women at the University of California, Berkeley, between 1999 and 2000 were found to be caused by identical strains of E. coli, which, the authors wrote, could have arisen after the women ate contaminated food.
In recent years the CDC has successfully identified the source of contamination in large foodborne disease outbreaks only about half the time. But the origins of slow-brewing infections are far more challenging to pinpoint. Even if the sausage I ate that morning was contaminated with drug-resistant COPs, I would never know it. If I got a serious infection months later, I could never prove that it came from this breakfast. I would probably never even think about this breakfast.
This is the crux of the problem: it is difficult, if not impossible, to trace resistant infections back in time to their microbial ground zeros. “It is a long way—geographically, temporally and in other ways—from the farm to the fork,” McEwen says. A hamburger can be made of meat from 100 different cows, so it is hard to pinpoint the one contributor that was contaminated. And scientists not only need to do that but also need to find out whether the way the animals were raised—whether or not they received antibiotics, for how long, at what dose and for what purpose—affected their bacteria in ways that could have spurred or worsened the outbreak. Industries also argue that farm bacteria only pose a risk to those working and living nearby, not the general public—which is why scientists try to get onto farms, to compare bacteria there with what ails the larger population.
Yet no one is gathering this kind of information. “There are very limited data collected at the farm level,” concedes Bill Flynn, deputy director for science policy at the FDA's Center for Veterinary Medicine. In September 2015 the FDA, the USDA and the CDC held a meeting in which they devised a plan to start collecting more on-farm data, but they did not receive the funding they requested to actually start doing it. In fact, for fiscal year 2016, the FDA received none of the $7.1 million it requested to study antibiotic resistance in animals.
Academic scientists are desperate to go on farms and study farm animals, too, but they are rarely granted access unless they have connections. When Smith hoped to collect samples from industrial turkey farms, she contacted every single registered turkey farm in Iowa. “None of them let us on,” she recalls. To study hog bacteria, Price and his colleagues have resorted to buying pig snouts at North Carolina butchers and swabbing them for bacteria because they cannot get to live animals. And remember that study in which Johns Hopkins researchers tailed chicken delivery trucks in cars? They had to conduct the study like that because they had no other way of getting close to the chickens—the researchers were not allowed on the farms.
It is not that livestock farmers are antiscience; it is that their employers, the meat companies, instruct them to keep outsiders away. A whopping 90 to 95 percent of U.S. poultry farmers and 48 percent of hog farmers (Beard being one) are contract growers—they sign contracts to raise animals for large companies like Tyson Foods, Smithfield Foods or Perdue Farms. Farmers are beholden to these companies because they undertake a huge amount of debt to start their business—a new poultry or hog farm costs a farmer about $1 million—yet they do not earn any money without a company contract; often farmers have only one choice of employer because a single company operates in their area.
Yet these company contracts—SCIENTIFIC AMERICAN obtained a recent contract from a former grower for Pilgrim's Pride, the largest chicken producer in the U.S.—contain clauses about animal protection, instructing farmers to “limit the movement of non-essential people, vehicles and equipment” around the farms. My visit to Beard's and Schoettmer's farms was preapproved by the National Pork Board. But when West Virginia poultry grower Mike Weaver invited a journalist onto his farm several years ago and his employer found out, “I was forced to attend ‘biosecurity retraining’ and was delayed receiving a new flock an extra two weeks, which amounts to a loss of revenue of around $5,000 for me,” he says. Price, as a scientist, convinced a handful of farmers to grant him farm access years ago, but then, he recalls, they “lost their contracts.”
Despite repeated requests, the American Farm Bureau Federation, the farm industry trade group, and Smithfield Foods, the world's largest hog producer and pork processor, declined to comment for this article and address the issue of whether industries were keeping scientists off farms.
Whatever the reason, the lack of data has made it easier for industry to fight regulations. In 1977, soon after Levy's study was published, the FDA announced that it was considering banning several antibiotics from animal feed over safety concerns. In the 39 years since, the industry has fought hard against these plans by arguing there was no definitive proof of harm. These arguments ultimately caused the FDA to change tactics, Flynn says, and to pursue the voluntary guidances instead.
But the disease-control exemption is a gaping hole in the guidances, many complain. “Do I think the total volume of antibiotic use will go down? I absolutely do not,” says H. Morgan Scott, a veterinary epidemiologist at Texas A & M University. In fact, antibiotic sales to farms have increased each year since the draft guidances were announced. In 2014 the nonprofit Pew Charitable Trusts analyzed the drug labels of all 287 antibiotic products that will be affected by the guidances and found that farmers will still be able to administer one quarter of the drugs at the same dosages and with no limits on treatment duration—as long as they say they are using them to prevent or control disease. Even the Animal Health Institute's Carnevale says the FDA guidances “could change the overall picture of how [antibiotics] are used, but whether [they're] going to affect total quantities of antibiotics remains to be seen.”
The requirement for veterinary prescriptions may not put a dent in antibiotic use, either. Many veterinarians prescribe and sell antibiotics for a profit or work closely with the food or pharmaceutical industries. A 2014 Reuters news investigation reported that half of all the veterinarians who advised the FDA on antibiotic use in food animals in recent years had received money from drug companies. “There are a lot of veterinarians who are attached to industry, who have a conflict of interest and who are beholden to the large producers—so they are inclined to go along with the status quo,” James Johnson says.
Several members of the U.S. Congress, including New York State Representative and microbiologist Louise Slaughter, have introduced bills to more tightly regulate antibiotic use on farms. Slaughter has pushed for her Preservation of Antibiotics for Medical Treatment Act for more than a decade. It has been supported by 454 organizations, including the American Medical Association. But after being referred to the Health subcommittee of the House Energy and Commerce Committee, the bill never reaches a vote.
One committee member who does not support the bill at this point, Representative Tim Murphy of Pennsylvania, has gone on record warning against the continued low-level use of antibiotics in food animals and the dangers that resistant bacteria pose to our food supply, says his press secretary, Carly Atchison. But he does not think the bill “strikes the appropriate balance needed in the use of medically important antibiotics in agriculture and farming,” Atchison says. There is also significant opposition to the bill from industry. The National Chicken Council spent $640,000 in 2015 to lobby, in part, against antibiotic-related legislation, and the Animal Health Institute spent $130,000, according to records from the nonprofit Center for Responsive Politics. Center data also show that veterinary pharmaceutical companies or livestock farming organizations have made campaign donations of more than $15,000 to more than half of the members of the Health subcommittee. “The trade organizations have been down there saying, ‘You can't show it's us—that we're causing the resistance,’” says Patty Lovera, assistant director of the nonprofit Washington, D.C.–based Food and Water Watch. “That has really gummed up the works for a long time.”
A Small Solution
After I left Beard's farm, I drove two hours to my final destination: Seven Sons Farms in Roanoke, Ind., which raises pigs on pastures and woodlands without antibiotics. A decade ago Seven Sons was a lot like the two farms I had just seen—it raised 2,300 hogs a year for Tyson Foods, regularly using drugs. But the family was worried about health effects, so it made some changes. In 2000 Seven Sons became what it calls a regenerative diversified farm, and today it raises about 400 pigs, 2,500 egg-laying hens and 120 forage-fed cattle on 550 acres of pasture.
Blaine Hitzfield, the second of the farm's seven namesake sons, took me on a short tour. I saw fewer than a dozen hogs lounging around a half-acre expanse of dirt and grass. Hitzfield did not ask me to wear coveralls, and he was not concerned that I had come directly from another hog farm. The animals on his farm, he explained, are hardier than those raised in confinement: not only do they have more space and mobility, but they are also weaned later so that they develop stronger immune systems. Nature helps as well. “The sun is a wonderful sanitizer, and the mud does wonders for keeping the parasites off,” he said. (If a pig does get sick, Seven Sons treats it with antibiotics but then sells it at auction rather than with their label.) His claims have research behind them. In a 2007 study, Texas Tech University researchers reported that pigs that had been raised outside had enhanced activity of bacteria-fighting immune cells called neutrophils when compared with animals raised inside.
Hitzfield conceded that Seven Sons' approach could be hard to envision as the future of industrial farming. “Conventional-minded farmers would say, ‘This is ridiculous; it would never work; it's not scalable’—and to a certain extent they're right,” he said. Seven Sons is just a small prototype, but Hitzfield said that with time and more research, much bigger versions would be possible. “On a per-acre basis, we're much more productive than we've ever been,” he added.
Some industrial farms are making changes, thanks in large part to consumer demand. They are not becoming small, diversified operations. But in February 2016 Perdue Farms announced that two thirds of its chickens would be raised without medically important antibiotics; Tyson Foods has pledged to stop using human antibiotics to raise its U.S. chickens by September 2017. Broiler chickens are far easier to industrially raise without antibiotics than pigs, cows or turkeys because they are slaughtered at younger ages.
But demand is driving some large-scale pork producers to scale back, too. “It is not an easy thing to do,” says Bart Vittori, vice president and general manager for pork at Perdue Farms' food division, which has an arm called Coleman Natural Foods. Coleman raises pigs on a vegetarian, antibiotic-free diet. “The demand is out there. Our consumers are smarter than ever, more informed than ever, asking more questions than ever,” Vittori says. The meat that comes out of Niman Ranch, a network of more than 725 family-run hog, lamb, cow and egg-laying hen farms throughout the U.S., has also been raised without drugs.
Products from Coleman, as well those from niche farms such as Seven Sons and Niman Ranch, are out of the financial reach of many Americans today. But the more that consumers demand antibiotic-free meat, the more supply there will be and—if basic economics holds true—the less it will cost.
Scientists still have many, many questions about antibiotic resistance—questions that may never get answered if food companies continue to ban outsiders from their farms. Even so, the weight of the evidence points strongly toward reducing antibiotic use on farms, relying instead on novel infection-control regimens or age-old strategies such as providing animals with ample space. Until some of those changes occur, researchers and the rest of us will continue to worry about the growing strength of foodborne bacteria and the increasing weakness of our medicine against them.

